European Rare Blood Disorders Platform (ENROL)
What is ENROL?
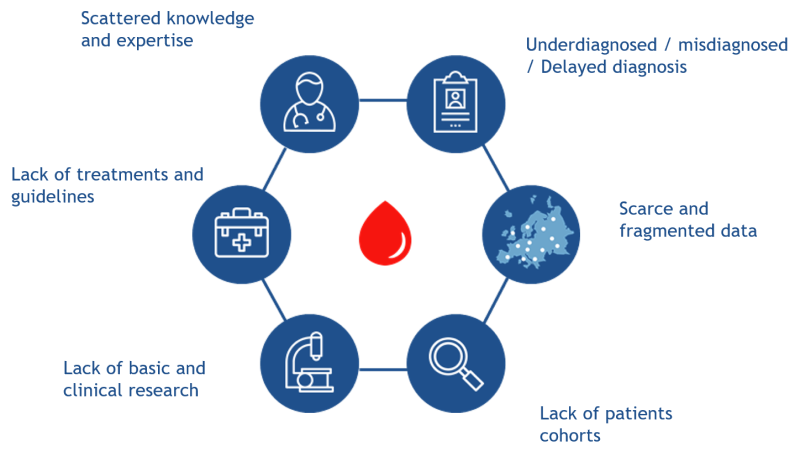
As for other rare diseases, bringing together sufficient data regarding patients affected by Rare Hematological Disorders (RHDs), especially less prevalent ones, is challenging. Multiple stakeholders perceive this scarcity and fragmentation of data as a relevant subject:
- Patients feeling isolated and in many occasions without diagnosis or late and with no options for curative treatments.
- Researchers and clinicians unable to reach critical numbers for engaging basic and clinical research.
- Health authorities lacking of epidemiological and disease burden data for the allocation of resources for best health planning.
In addition, underrepresentation of ultra-rare RHDs in coding systems determines a general difficulty in tracing RHDs patient pathways within healthcare systems. This untraceable data also hampers the long-term sustainability of existing and new patient registries established at both the national and the European levels.
Accordingly, the European Commission launched the European Platform on Rare Disease Registration (EU RD Platform) aiming to cope with the fragmentation of rare diseases patient data contained in hundreds of registries across Europe.
With regards to RHDs, there are some examples of existing European networks working on development and maintenance of patient registries, such as EBMT (European Society for Blood and Marrow Transplantation), MDS-RIGHT (Providing the right care to the right patient with MyeloDysplastic Syndrome at the right time), EuroHistioNet (Reference network for Langerhans Cell Histiocytosis and associated syndromes), or RADeep (Rare Anaemia Disorders European Epidemiological Platform). However, there is no common accessible repository of existing national and European registries on RHDs at the European Union (EU) level or information on the type of data gathered and clinical outcomes.
The lack of uniform standards for data collection has led to the implementation of a large number of patients' registries through different approaches, gathering non-comparable and fragmented data, which hamper collaborative projects and generation of enough robust evidence for translation of results into clinical practice.
ENROL, the European Rare Blood Disorders Platform, has been conceived in the core of ERN-EuroBloodNet as an umbrella for both new and already existing registries on RHDs. ENROL aims at avoiding fragmentation of data by promoting the standards for patient registries' interoperability released by the EU RD platform.
ENROL's principle is to maximize public benefit from data on RHDs opened-up through the platform with the only restriction needed to guarantee patient rights and confidentiality, in agreement with EU regulations for cross-border sharing of personal data.
Accordingly, ENROL will map at the EU level demographics, survival rates, diagnosis methods, genetic information, main clinical manifestations and treatments in order to obtain epidemiological figures and identify trial cohorts for basic and clinical research. To this aim, ENROL will connect and facilitate upgrading of existing RHD registries, while promoting the building of new ones when / where lacking. Target-driven actions will be carried-out in collaboration with EURORDIS for educating patients and families about the benefits of enrolment in such registries, including different cultural and linguistic strategies.
The standardised collection and monitoring of disease specific health care outcomes through the ENROL user-friendly platform will determine how specialised care is delivered, where are the gaps in diagnosis, care or treatment and where best to allocate financial, technical or human resources. Moreover, it will allow promoting research especially for those issues that remain unanswered or sub optimally addressed by the scientific community; furthermore, it will allow promoting clinical trials for new drugs. ENROL will enable the generation of evidence for better healthcare for RHD patients in EU as ultimate goal.
ENROL has officially started 1st June 2020 with a duration of 36 months. ENROL is co-funded by the Health Programme of the European Union under the call for proposals HP-PJ-2019 on Rare disease registries for the European Reference Networks. GA number 947670.
ENROL online Kick off meeting was successfully held last 2nd July 2020 with more than 120 registrations. Download here the main outcomes and slides presented during the meeting
