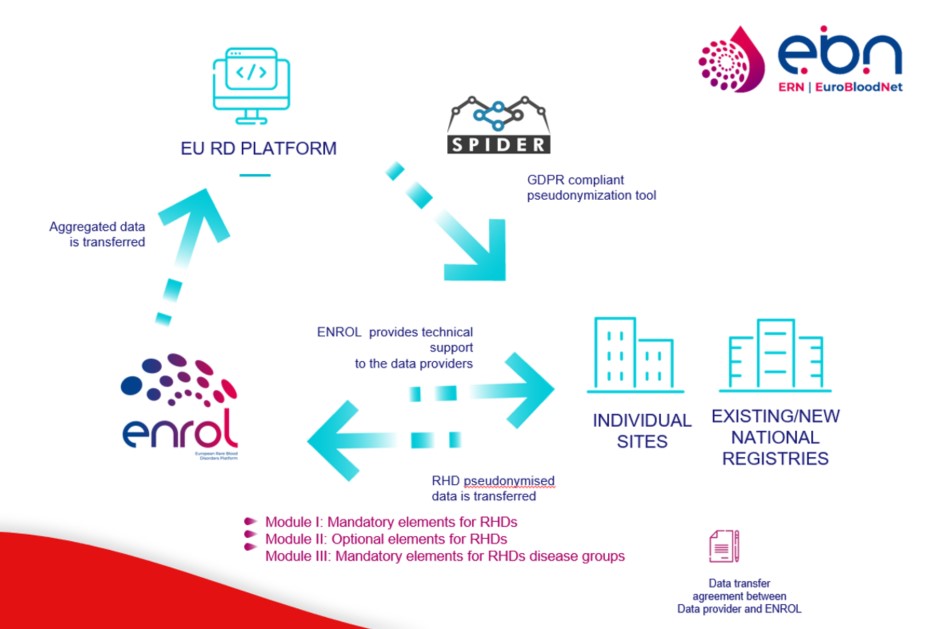
ENROL strategy for data gathering combines the exhaustiveness of data collection at the EU level for health planning and epidemiological purposes, with a higher level of RHDs data granularity for promoting research and identification of patients' groups.
Accordingly, the ENROL Registry platform has been designed to integrate different types of data from any available sources:
a) counts/aggregated level data
b) pseudonymised individual patient-level data
Pseudonymisation of personal data will be performed by SPIDER , the GPDR-compliant tool offered by the EU-RD Platform in the context of rare disease registries.
ENROL dataset has been developed in a bottom-up design with three levels of depth.
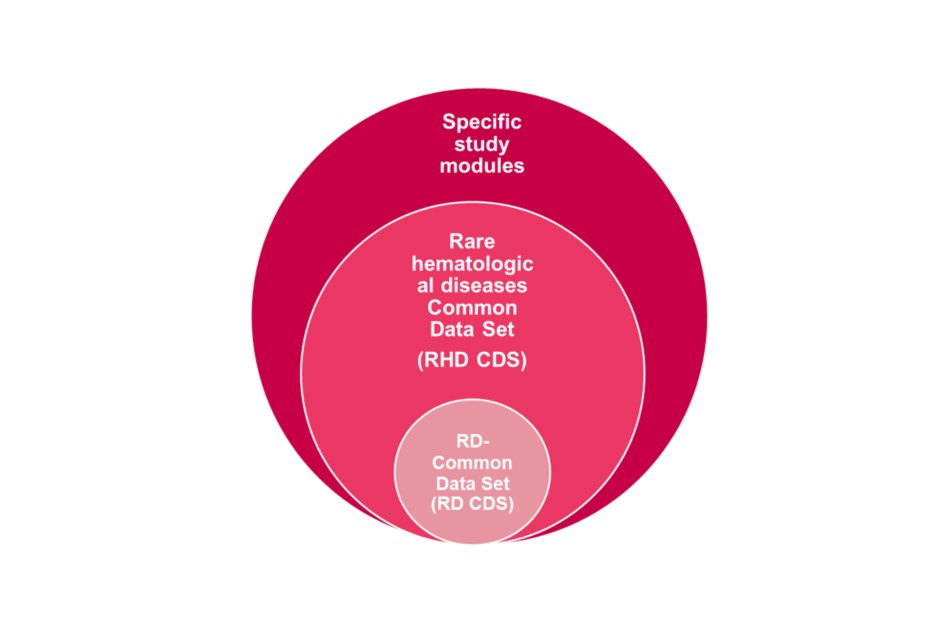
ENROL dataset integrates multiple codifications and international standards in order to ensure interoperability with available sources:
Find here ENROL recommendations on codification schemes for rare hematological diseases as an initial set of data standardization principles to ensure interoperability with ENROL.
The common data model for Interoperability platforms will be based on OMOP.
RHDs patients' data will be collected through ENROL platform from any EU-MS country combining different sources of data, including a) Healthcare providers' (HCPs) hospital records and b) Existing registries.
The Help section of ENROL provides an overview of the scope and main procedures of ENROL and, also, facilitates the user in the registration/addition/editing of patient data.
The next steps for promoting e-Health records in ERN-EuroBloodNet members include a mapping of the availability of the RHD-CDS elements in the EHR and the use of related codifications e.g. ORPHA.